I’m a vegetarian, and while you may assume, therefore, that I eat a lot of salads, that’s not actually the case. I do eat lots of cooked greens, though, and lots of other vegetables, of course. Unfortunately, they often end up turning a muddy green color, which makes them look much less appetizing (though I still eat them because I know how good they actually taste). What makes them green? And why do greens sometimes turn brown or muddy-colored upon cooking? How can we prevent it?
Why are greens green?
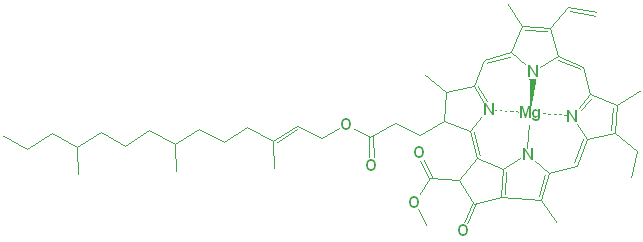
Plants generate their energy by consuming carbon dioxide (CO2) to generate sugars or carbohydrates. The plants use the energy in light to drive this reaction, so they need to absorb it somehow. The primary light-absorbing molecule is chlorophyll, and though there are many forms, chlorophyll a and b are the most common in land plants. Although chlorophyll a is more common, their chemical structures are very similar.
Chlorophyll isn’t located everywhere in the cell, but is instead concentrated into special plant cell organelles called chloroplasts. The chloroplasts have their own small structures inside, but everything is contained within a double membrane.
Why don’t greens stay green?
If the chlorophyll and chloroplasts are responsible for a plant’s green color, then degradation of the chlorophyll will lead to a decline in the green color.
During heating, the structure of the leaf collapses, and gas in the leaf escapes. Initially, this brightens the green color, as the gas serves to “cloud” or distort the green color. However, as the gas escapes, some of the internal structures of the leaf are broken down, which disrupts the double membranes of the chloroplast.
Although ordinarily chlorophyll is not water soluble, the heat can also cause degradation and can cause the long hydrocarbon chain to react and fall off. Once the chlorophyll loses the hydrocarbon chain, it becomes much more water-soluble and will escape into the cooking liquid. This serves primarily to dilute the green color of the leaf.
The damage to the hydrocarbon tail can be enabled by acidic or alkaline conditions, as well as accelerated, or catalyzed, by an enzyme that is most active at elevated temperatures. Boiling, however, will destroy the enzyme, halting further chlorophyll degradation through that pathway.
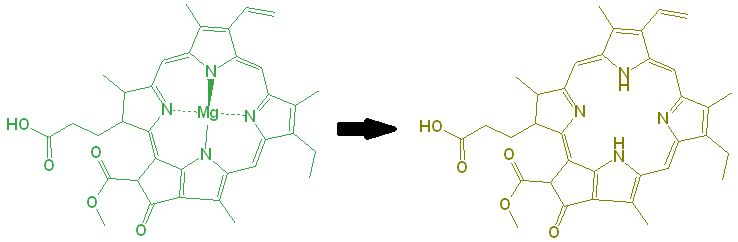
There is a second degradation pathway. The magnesium (Mg) in the center is also responsible for the bright green color of the chlorophyll. Under even slightly acidic conditions, the Mg can be displaced by hydrogen ions, and the resulting molecule is an olive green color.
To prevent this, you can try to avoid cooking the greens in water, by stir-frying them, but as the cell structure collapses, other acids from within the cell will be released, causing the displacement of Mg anyway.
So how can we keep vegetables green?
We have a few options. Cook the greens in an alkaline solution, instead of an acidic solution. The easiest way to do this is by adding a little bit of baking soda to the cooking water. An alkaline solution will keep the concentrations of hydrogen ions in solution very low, making the displacement difficult. If you add too much baking soda, however, and make the solution too alkaline, you can start to break down the vegetables and they may taste soapy (alkaline solutions are used in making soap in general).
You can also try replacing the Mg with other metals that bond more strongly so that the acid doesn’t displace them. Copper (Cu) and zinc (Zn) can do this, however, consuming hundreds of milligrams of Zn a day or grams of copper a day are harmful to humans.
So what else can you do? Restrict the amount of time that you cook the greens from between 5-7 minutes. Use a lot of water so that any acid that is generated by the collapse of the leaf structure is diluted. pH is a function of concentration of the acidic group, so by adding a lot of water, the pH is affected much less. After cooking, you can stop any further damage by rapid cooling with ice water. Finally, any acidic sauces or dressings should be added right before consumption or serving.
Sources: On Food and Cooking by Harold McGee
https://en.wikipedia.org/wiki/Zinc_toxicity
https://en.wikipedia.org/wiki/Copper_toxicity

One response